Sarmscentral is a leading online retailer specializing in high-quality Selective Androgen Receptor Modulators (SARMs). Our mission is to provide athletes, bodybuilders, and fitness enthusiasts with the best possible products to help them achieve their goals.
- SARMS
- SERMS & PCT
About
View MoreSarmscentral is a leading online retailer specializing in high-quality Selective Androgen Receptor Modulators (SARMs). Our mission is to provide athletes, bodybuilders, and fitness enthusiasts with the best possible products to help them achieve their goals.
Latest Blog

- PROHORMONES
About
View MoreSarmscentral is a leading online retailer specializing in high-quality Selective Androgen Receptor Modulators (SARMs). Our mission is to provide athletes, bodybuilders, and fitness enthusiasts with the best possible products to help them achieve their goals.
Latest Blog

- AAS
About
View MoreSarmscentral is a leading online retailer specializing in high-quality Selective Androgen Receptor Modulators (SARMs). Our mission is to provide athletes, bodybuilders, and fitness enthusiasts with the best possible products to help them achieve their goals.
Latest Blog

- PEPTIDES
About
View MoreSarmscentral is a leading online retailer specializing in high-quality Selective Androgen Receptor Modulators (SARMs). Our mission is to provide athletes, bodybuilders, and fitness enthusiasts with the best possible products to help them achieve their goals.
Latest Blog

- NOOTROPIC
About
View MoreSarmscentral is a leading online retailer specializing in high-quality Selective Androgen Receptor Modulators (SARMs). Our mission is to provide athletes, bodybuilders, and fitness enthusiasts with the best possible products to help them achieve their goals.
Latest Blog
Nothing Found.
Apologies, but no results were found for the requested archive.

- OTHER COMPOUNDS
About
View MoreSarmscentral is a leading online retailer specializing in high-quality Selective Androgen Receptor Modulators (SARMs). Our mission is to provide athletes, bodybuilders, and fitness enthusiasts with the best possible products to help them achieve their goals.
OTHER COMPOUNDS
Latest Blog

In today’s super-fast world, being sharp and focused is key. It can be tough to keep up, let alone excel. But don’t worry, we’ve got your back. There’s this awesome thing called Ibudilast that’s like a magic key to unlocking your brain’s full potential. It helps you focus better, remember stuff, and think faster. It’s like having superpowers for your brain!
If you’re looking for a way to step up your game, give Ibudilast a try. Just incorporate it into your daily routine and watch your mind soar to new heights. You’ll be amazed at what you’re capable of when you unleash all that hidden brilliance inside you. It’s like finding a hidden treasure trove of awesomeness just waiting to be discovered!
The Brain Boosting Powerhouse: How it Works?
Ibudilast is a serious powerhouse when it comes to boosting brain power. It does this by giving your brain a nice dose of stuff called neurotrophic factors. These little helpers, like BDNF (Brain-Derived Neurotrophic Factor), help your neurons grow and stay healthy. When you take Ibudilast, it makes your focus sharper, improves your memory, and gives you a serious mental edge. [R]
But that’s not all! Ibudilast also has some cool anti-inflammatory effects. This means it can help reduce brain inflammation, which can mess with your thinking. So not only does it give you a short-term brain boost, but it also helps protect your noggin in the long run. With Ibudilast by your side, you’ll feel mentally clear and on top of your game like never before! [R]
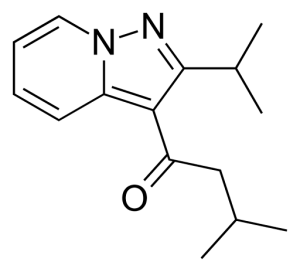
Image Source: Wikipedia
At the heart of Ibudilast’s brain perks is its ability to tame inflammation in the noggin. Research shows that it can bring down the swelling that can hurt brain cells and lead to memory problems and brain diseases. By calming inflammation, Ibudilast helps protect brain cells and keeps our minds sharp.
Another cool thing about Ibudilast is that it can make our brains more flexible. This means it helps us learn new stuff faster and makes us more creative. It’s like giving your brain a workout that keeps it young and ready to take on new challenges.
In summary, Ibudilast is like a little superhero for our brains. It fights inflammation, protects brain cells, and gives our brains a boost in flexibility. So if you’re looking for a natural way to keep your brain sharp, Ibudilast might just be the ticket. [R]
Benefits of Ibudilast:

- Provides Prophylaxis Against Asthma:
Ibudilast has anti-inflammatory properties that can help manage asthma symptoms. It reduces airway inflammation and hyperresponsiveness, which can lead to improved breathing and reduced frequency of asthma attacks. [R]
- Facilitates the Eradication of Opioid Dependence:
Ibudilast is being studied for its potential to assist in the treatment of opioid dependence. It may help reduce cravings and withdrawal symptoms by modulating neuroinflammation and enhancing the efficacy of other treatments used in addiction management. [R]
- Mitigates Post-Stroke Vestibular Dysfunction:
Ibudilast can be beneficial for patients experiencing dizziness and balance issues following a stroke. Its neuroprotective and anti-inflammatory effects may help improve neurological function and reduce symptoms of post-stroke dizziness. [R]
- Confers Protection Against Multiple Sclerosis:
In the context of multiple sclerosis, Ibudilast has shown promise in reducing the progression of the disease. It works by decreasing inflammation and protecting against neuronal damage, potentially slowing the advancement of MS and alleviating some symptoms.[R]
- Suppresses Symptoms of Allergic Rhinitis (Hay Fever):
Ibudilast’s anti-inflammatory properties also make it effective in managing hay fever. It can help reduce nasal inflammation, congestion, and other allergic symptoms, improving overall comfort during allergy seasons. [R]
- Offers Protection Against Allergic Conjunctivitis:
For individuals with allergic conjunctivitis, Ibudilast can provide relief by reducing inflammation and allergic reactions in the eyes. This can help alleviate symptoms such as itching, redness, and swelling. [R]
Incorporating Ibudilast into Your Daily Routine
Integrating Ibudilast into your daily routine is a transformative step toward enhancing your cognitive abilities. Consider taking Ibudilast in the morning with a healthy breakfast to kickstart your day with mental clarity and focus. Create a routine around your dosage to ensure consistency and maximize its benefits.
Pairing Ibudilast intake with brain-boosting activities like reading, puzzles, or meditation can amplify its effects. Embrace the ritual of incorporating Ibudilast into your daily regimen as a moment of self-care and empowerment. By weaving this nootropic into your routine, you are setting yourself up for success in achieving mental sharpness and cognitive excellence.
Personal Success Stories with Ibudilast
image source: Reddit
Overcoming Challenges with Ibudilast
Ibudilast may present challenges along the way, but perseverance is key. Adapting to potential side effects such as mild headaches or digestive issues can be managed through proper hydration and adjusting dosage levels gradually.
Staying informed and seeking guidance from healthcare professionals can provide valuable insights on optimizing the benefits of Ibudilast while mitigating any obstacles that may arise. Remember, each challenge overcome by you brings you one step closer to unlocking your full cognitive potential.
The Future of Nootropics: Ibudilast Leading the Way
As we look ahead to the future of cognitive enhancement, Ibudilast emerges as a pioneering force in the realm of nootropics. With its potent neuroprotective and anti-inflammatory properties, Ibudilast is poised to revolutionize the way we optimize brain function and cognitive performance. Researchers are increasingly fascinated by the untapped potential of this compound, fueling optimism for its groundbreaking advancements in enhancing mental clarity and focus.
Through ongoing research and clinical trials, Ibudilast is paving the way for a new era of cognitive enhancement, offering hope for individuals seeking improved memory, concentration, and overall brain health. As this innovative nootropic gains traction in scientific communities worldwide, it holds the promise of unlocking greater cognitive abilities and empowering individuals to reach new heights of mental acuity and intellectual prowess.
Why Purerawz is the Best Place to Buy Ibudilast
When it comes to purchasing high-quality nootropic supplements like Ibudilast, purerawz.co stands out as a beacon of excellence. Their commitment to purity and potency is unmatched in the industry, ensuring that you receive the most effective product for enhancing cognitive function.
With a reputation for transparency and integrity, Purerawz sources only the finest ingredients for their products, guaranteeing optimal results. When you choose Purerawz as your supplier of Ibudilast, you can trust that you are investing in your brain health with a product that meets the highest standards of quality and efficacy.
FAQs
Is Ibudilast safe for long-term use?
When taken as directed, Ibudilast is generally considered safe for long-term use. However, individual responses may vary, so it’s important to consult with a medical professional before starting any new supplement regimen.
Can Ibudilast be used in combination with other nootropics?
While Ibudilast can be effective on its own, some users have reported enhanced benefits when combining it with other nootropics. It’s crucial to research potential interactions and start with lower doses to gauge how your body responds to the combination. Always prioritize safety and consult with a healthcare provider.
Are there any side effects of Ibudilast?
Ibudilast, while promising for various conditions, can have side effects. Common ones include gastrointestinal issues (nausea, vomiting, diarrhea), headaches, dizziness, and fatigue.
Conclusion
As we conclude our exploration of the incredible benefits of Ibudilast as a nootropic, it becomes abundantly clear that this innovative compound has the potential to revolutionize cognitive enhancement. With each dose of Ibudilast, you are not just supplementing your brain with an enhancer; you are investing in your future self, paving the way for sharper focus, enhanced memory retention, and heightened cognitive function. The journey towards maximizing your cognitive potential with Ibudilast is not just a path—it’s an exhilarating adventure filled with endless possibilities and boundless opportunities for growth.