The proper functioning of reproductive organs is of great significance for various reasons, encompassing both biological and psychological aspects. The primary function of reproductive organs is to facilitate biological reproduction. In both males and females, reproductive organs play a crucial role in the process of fertilization, leading to the development of a new individual and the continuation of the species. This article will cover a revolutionary supplement called triptorelin (GnRH) which is used for various purposes, primarily in the treatment of conditions related to hormone-sensitive organs such as the prostate, breast, and other reproductive system disorders.
Introduction
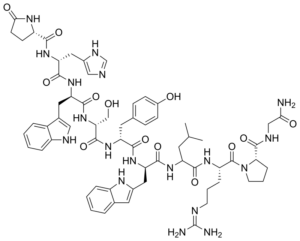
Triptorelin is a synthetic analog/gonadotropin-releasing hormone agonist(GnRH). GnRH is a hormone that plays a crucial role in the regulation of reproductive functions. GnRH regulates the pituitary secretion of gonadotropins luteinizing hormone (LH) and follicular-stimulating hormone (FSH) from the pituitary gland. It has been proven to have the potential to induce a sustained decrease in FSH and LH levels. {R} The only change between GnRH and Triptorelin is the introduction of a residue of D-Triptophane instead of a glycine in position 6 amino acid.
Like other GnRH agonists, this research peptide, too, has shown to have the potential to suppress testosterone levels in men with prostate cancer, thereby stopping or preventing the growth of prostate cancer cells. In the EU, it is an approved treatment for advanced prostate cancer in males. {R}
Triptorelin Peptide – Product Information
Triptorelin peptide is designed to mimic the action of GnRH and has various medical applications. Triptorelin is available under various brand names, and the specific names may vary by region and pharmaceutical company. It is used in the treatment of several medical conditions, including prostate cancer and endometriosis. It can also be used to alleviate symptoms associated with endometriosis in women and is often employed to treat children who experience early puberty. {R}
In some cases, this product may be used in controlled ovarian stimulation protocols for in vitro fertilization (IVF) to prevent premature ovulation. Triptorelin is typically administered as an injection with the frequency and dosage dependent on the specific medical condition being treated and the physician’s recommendations.
Triptorelin (GNRH) – Mechanism of Action
GnRH is a hormone produced in the hypothalamus of the brain, and it plays a key role in the control of the menstrual cycle, fertility, and the production of sex hormones. As a GnRH agonist, the mechanism of action of triptorelin involves its interaction with the GnRH receptors in the pituitary gland. Here’s a step-by-step explanation of how triptorelin works:
Image Source: https://en.wikipedia.org/wiki/Triptorelin
Stimulation of Gonadotropin Release:
Triptorelin stimulates the release of luteinizing hormone (LH) and follicle-stimulating hormone (FSH) from the pituitary gland. {R}
Pulsatile Stimulation vs. Continuous Stimulation:
Normally, the release of GnRH from the hypothalamus occurs in a pulsatile manner. This pulsatile release of GnRH stimulates the pituitary gland to release LH and FSH. However, when triptorelin is administered, it initially stimulates the pituitary gland in a similar pulsatile fashion. {R}
Desensitization of GnRH Receptors:
With continued administration of triptorelin, the GnRH receptors in the pituitary become desensitized. This means that they become less responsive to the continuous presence of triptorelin. {R}
Suppression of Gonadotropin Release:
As a result of desensitization, the pituitary gland becomes less responsive to the pulsatile release of GnRH, leading to a decrease in the secretion of LH and FSH. {R}
Reduction in Sex Hormone Production:
The reduction in LH and FSH levels ultimately leads to a decrease in the production of sex hormones, including testosterone in men and estrogen in women. {R}
Moreover, in the context of specific medical applications, this mechanism of action has therapeutic implications. In the treatment of prostate cancer, the reduction in testosterone levels is beneficial since prostate cancer growth is often dependent on testosterone. The ultimate goal of triptorelin treatment is to modulate the hormonal environment to achieve therapeutic effects in various hormone-sensitive conditions.
Triptorelin Benefits
Triptorelin, as a peptide, has several medical applications, and its benefits depend on the specific condition being treated. Here are some of the benefits associated with the use of triptorelin in different medical contexts:
- Triptorelin has been approved in the EU as a potential adjuvant treatment of early-stage breast cancer in women. The potential therapy combines it with tamoxifen or an aromatase inhibitor (AI) {R}
- Triptorelin is often used to treat advanced prostate cancer as part of androgen deprivation therapy. By initially stimulating the release of luteinizing hormone (LH) and follicle-stimulating hormone (FSH) and then desensitizing the pituitary gland, triptorelin reduces the production of testosterone. Since prostate cancer growth is often dependent on testosterone, lowering its levels can help slow down the progression of the disease. {R}
- In the treatment of endometriosis, triptorelin is used to suppress ovulation and create a hypoestrogenic state. By reducing estrogen levels, triptorelin helps alleviate symptoms associated with endometriosis, such as pain and inflammation. {R}
- Triptorelin is used to treat central precocious puberty, a condition in which puberty begins too early in children. By modulating the release of LH and FSH, triptorelin can delay the onset of puberty and slow down the maturation process. {R}
- In the context of assisted reproductive technologies like in vitro fertilization (IVF), triptorelin is sometimes used to control the timing of ovulation. This allows healthcare providers to optimize the chances of successful fertilization and pregnancy during ART procedures. {R}
- Triptorelin treatment may be associated with side effects, but in some cases, it can also provide relief from symptoms. For example, in prostate cancer treatment, patients may experience a reduction in symptoms like hot flashes. {R}
Side Effects
Triptorelin, like any medication, may cause side effects. However, since it’s an approved drug, potential side effects associated with the use of triptorelin are mild and rare including hot flashes, decreased libido, and fatigue.
Some individuals may experience swelling or redness at the injection site or changes in bone density with long-term usage.
Recommended Dosage of Triptorelin:
The recommended dosage for the drug with optimum results is 0.75 mg intramuscularly (IM) every 4 weeks or 11.25 mg IM every 12 weeks. Moreover, 22.5 mg IM every 24 weeks can also be taken.
Is it Legal?
It is an FDA-approved treatment for central precocious puberty (a condition that causes early sexual development) in children aged 2 years and above. {R}
Moreover, this research peptide has also been approved as a treatment for endometriosis. Endometriosis is a condition in which tissue similar to those that line the woman’s uterus grows outside the uterus. {R}
Where to Buy Triptorelin Peptide Online?
We recommend PureRawz as the best place to buy Triptorelin. To be the best supplier of research chemicals, the online store provides reference materials with every product they sell. Purerawz sells Triptorelin in injectable form.